Case of the Month March – by Ena Holjevic
Clinical Presentation:
An 82-year-old male patient arrived at the emergency department with shortness of breath, a dry cough that improved with rest, and swelling of his left leg. A CT pulmonary angiography was performed to rule out pulmonary embolism, revealing no signs of thrombosis; however, bilateral pleural effusions were detected. Additionally, a CT scan of the abdomen and pelvis was performed, but no tumor mass was found. The clinicians performed a therapeutic pleural puncture and sent the pleural fluid for cytological analysis.
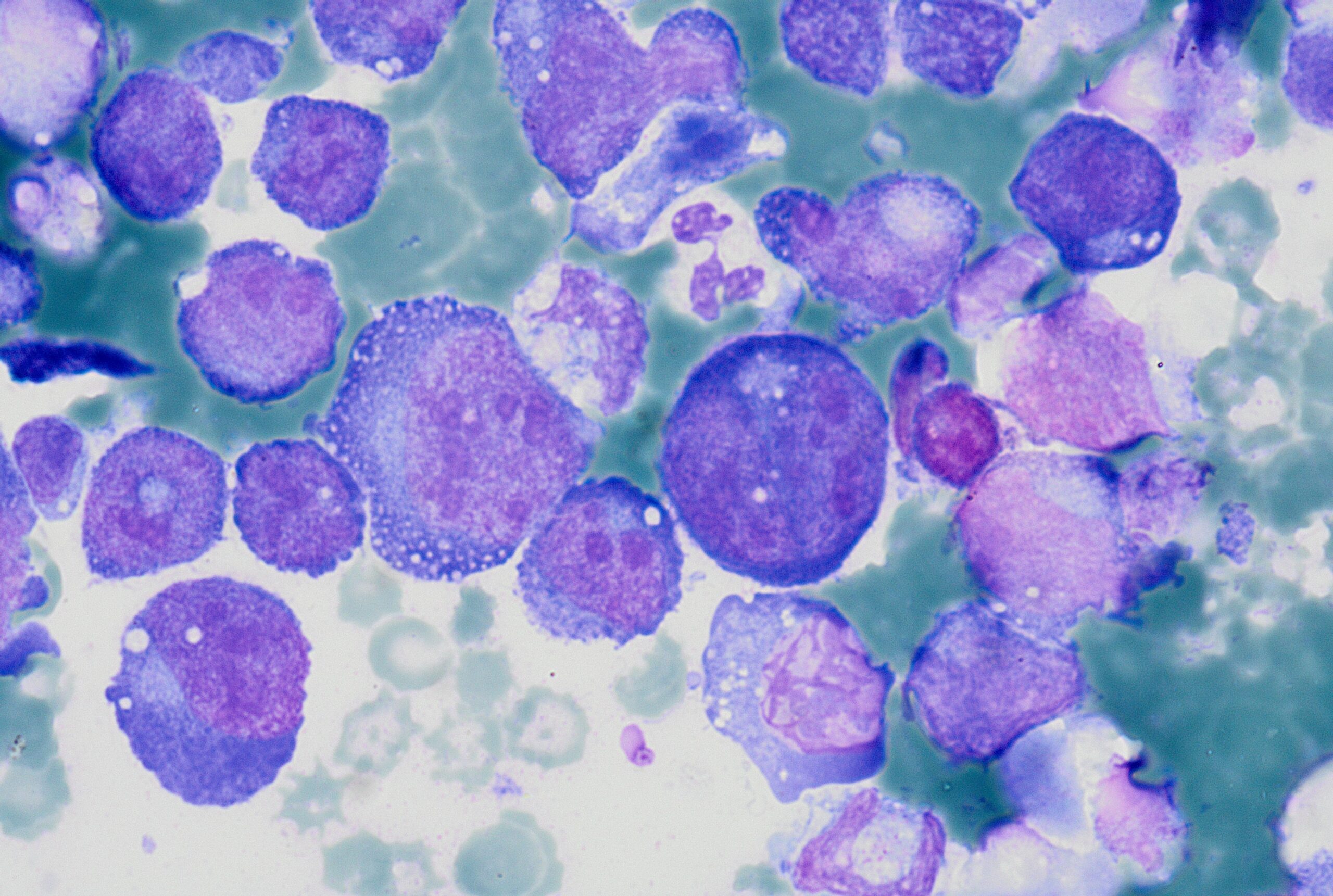
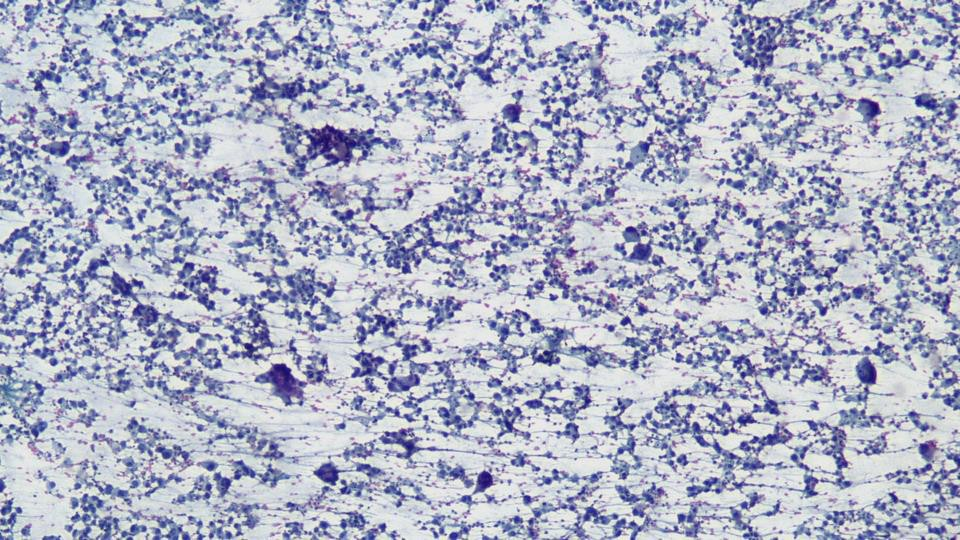
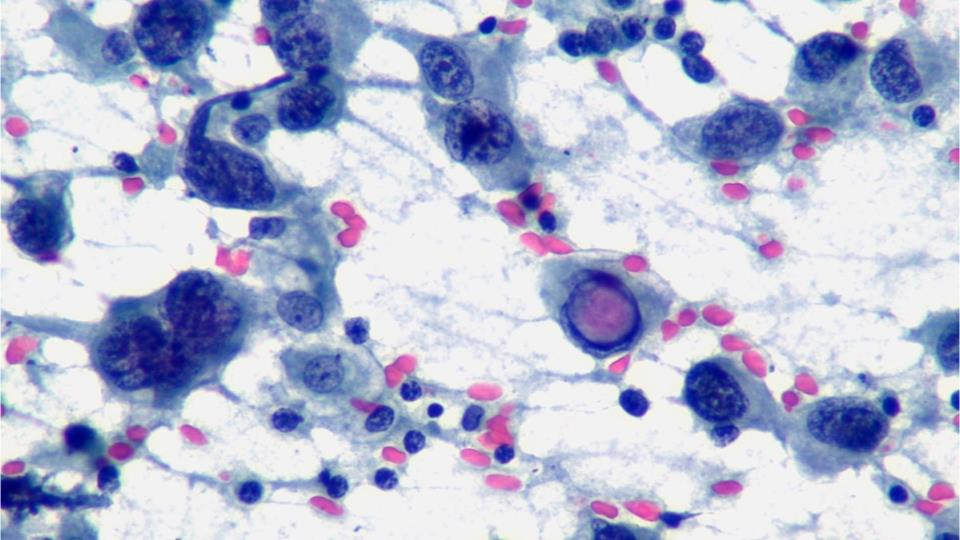
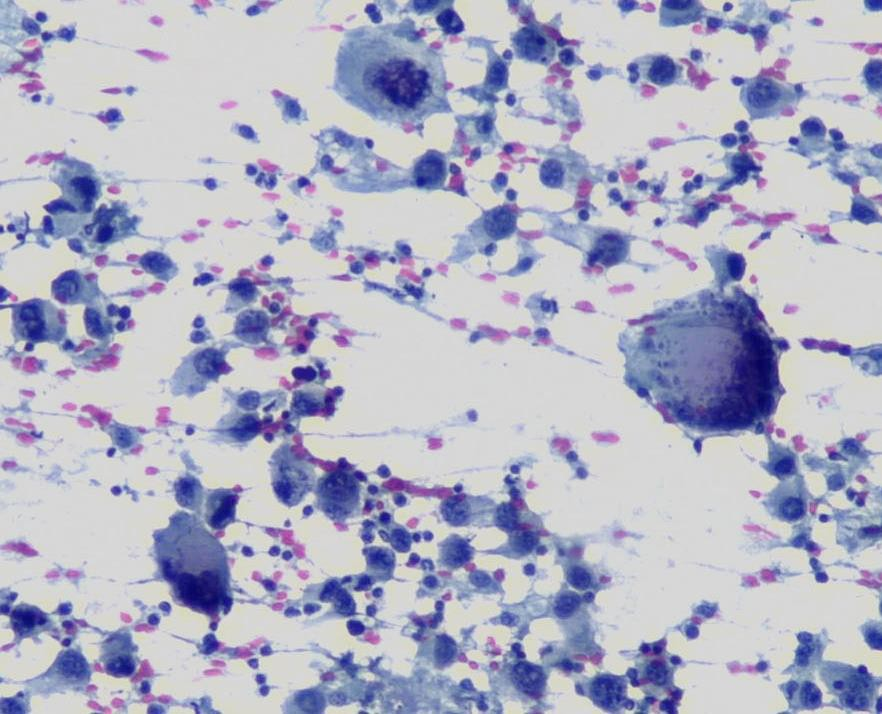
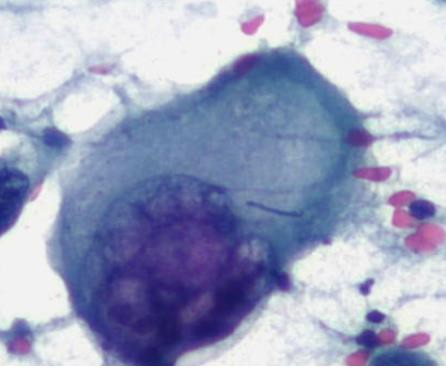
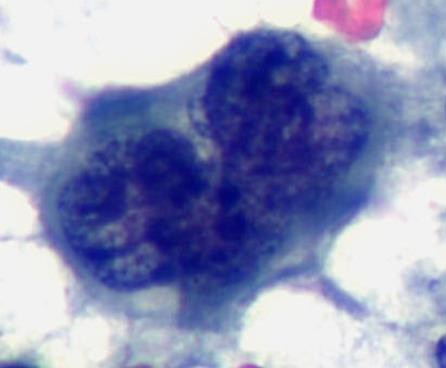
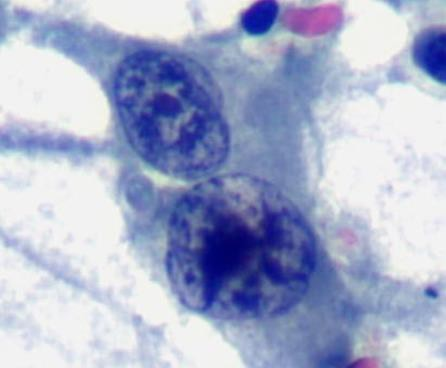
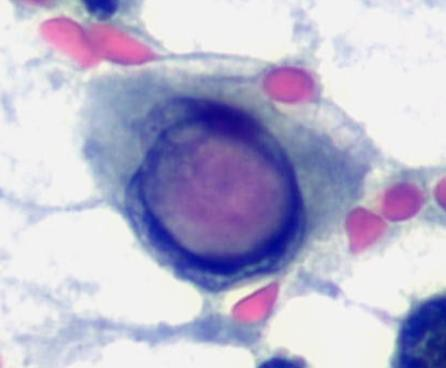
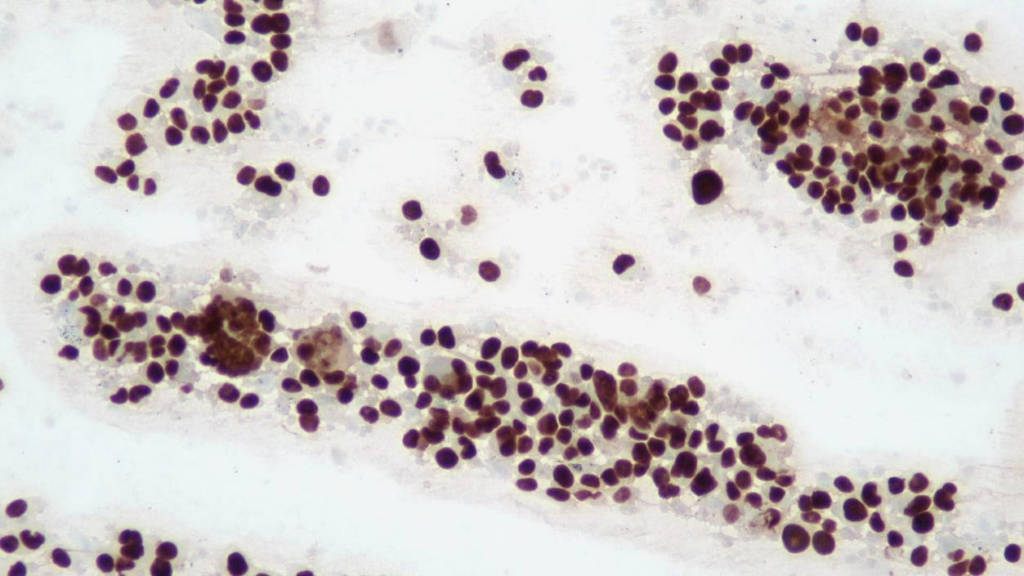
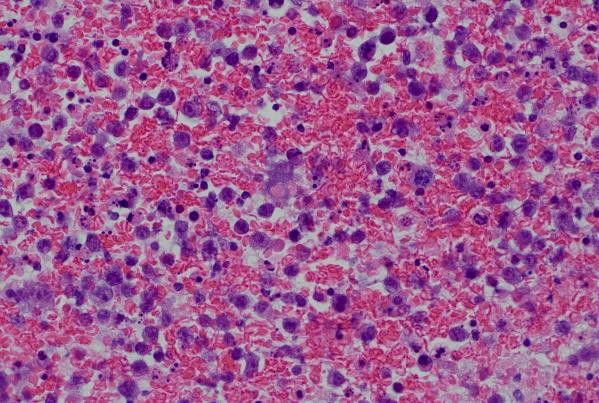
A cytological smear was prepared from the pleural fluid sediment. Microscopic examination revealed numerous large atypical lymphoid cells with irregular nuclear contours, prominent nucleoli, and abundant basophilic cytoplasm. Some cells displayed plasmacytoid features with occasional vacuolization.
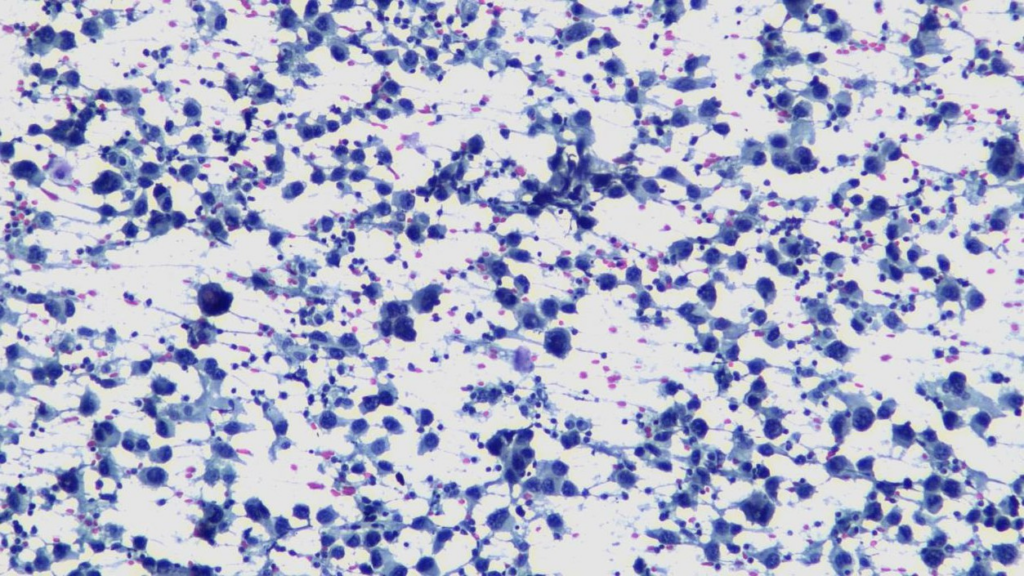
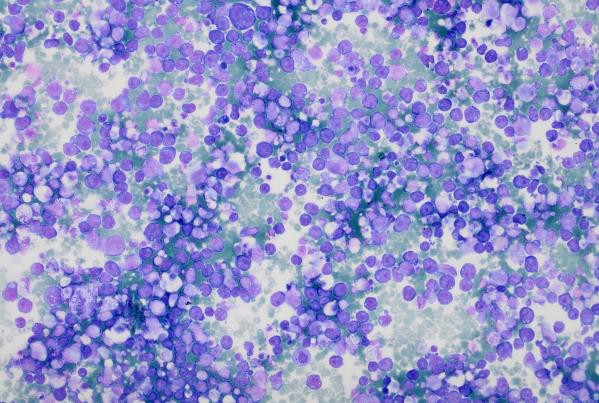
Highly cellular pleural fluid sediments showed large atypical lymphoid cells with prominent nucleoli and abundant cytoplasm.
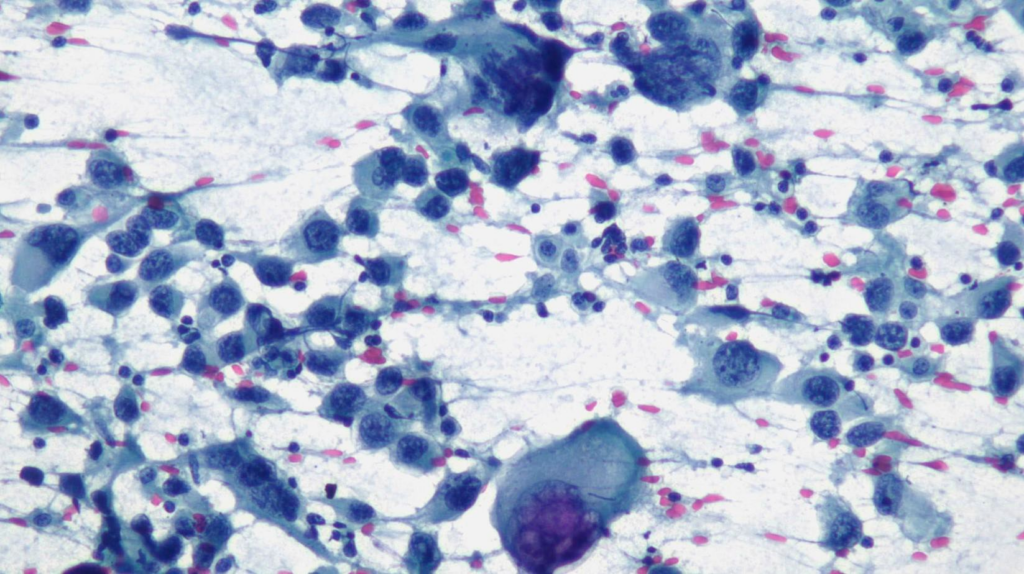
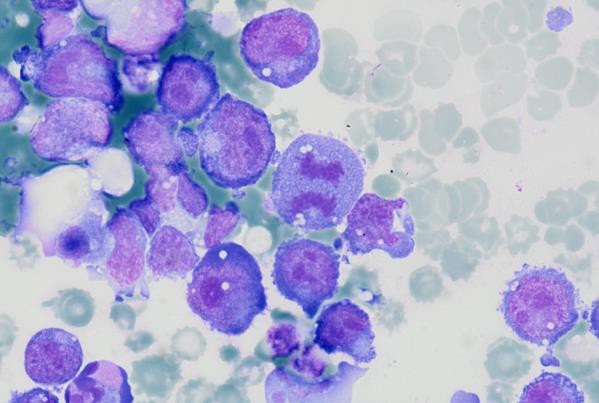
High magnification view highlighting plasmacytoid morphology and mitotic activity.
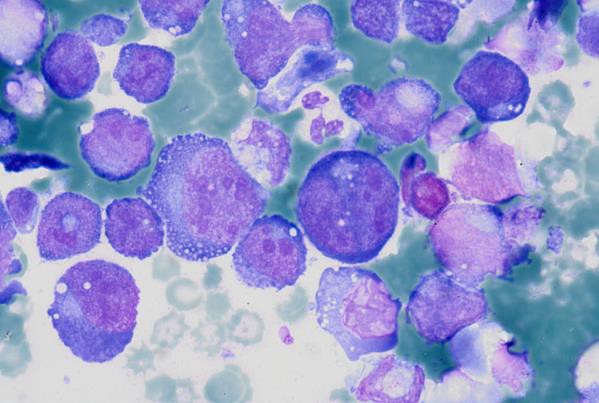
Prominent nucleoli, abundant basophilic cytoplasm.
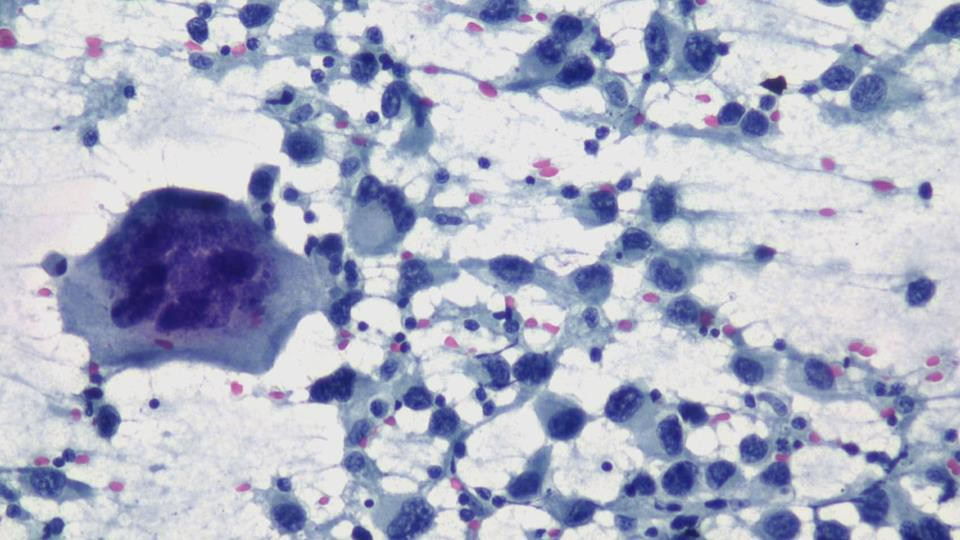
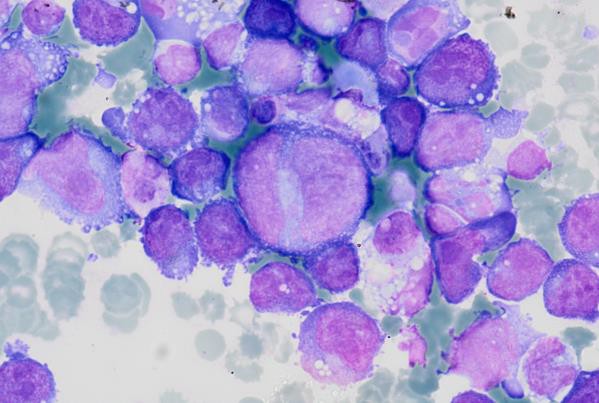
Cytoplasmic vacuoles, multinucleation.
A cell block was prepared from the pleural fluid sediment for further analysis.
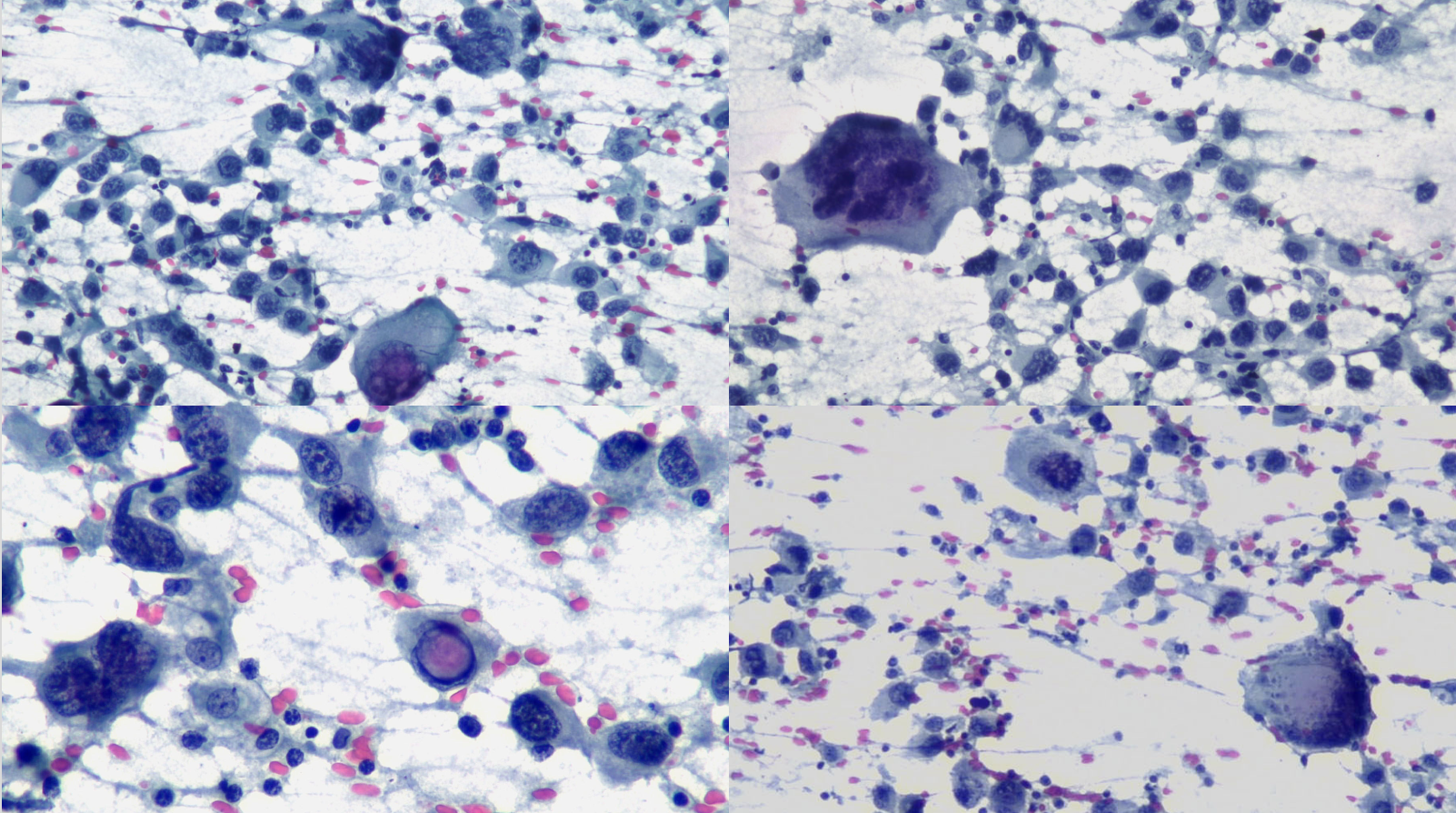
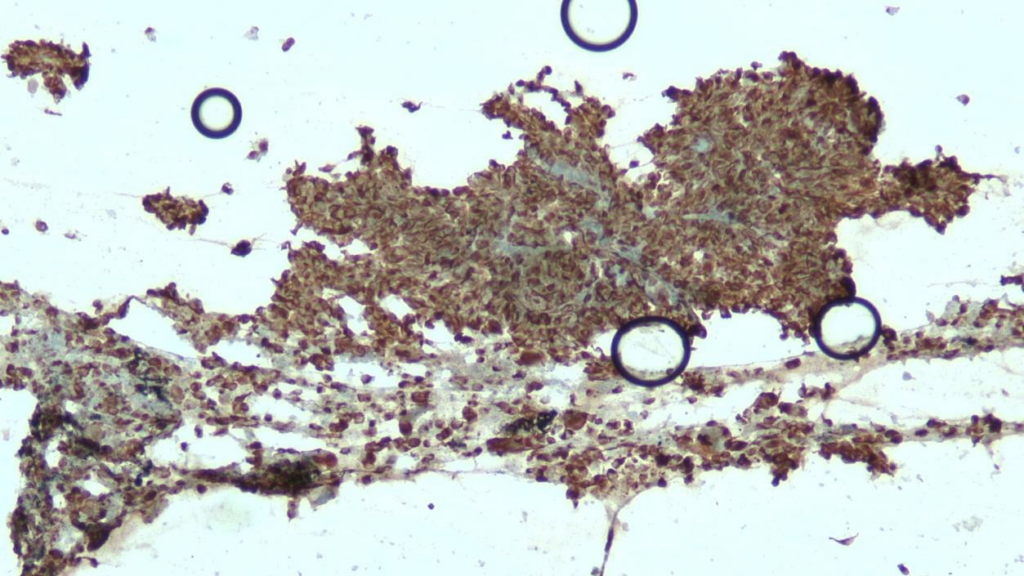
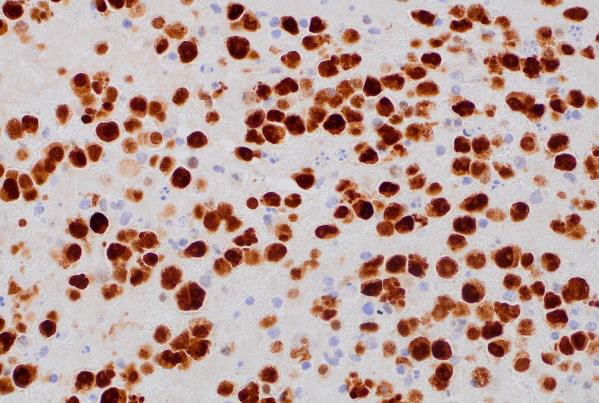
Immunohistochemical (IHC) staining was performed on the cell block sections to reveal the immunophenotype of the atypical lymphoid cells. The tumor cells were negative for CD20 and CD3, while showing positive staining for CD30, CD138, HHV-8 and MUM 1.
CD3 (200x magnification): Demonstrating T-cell marker expression.
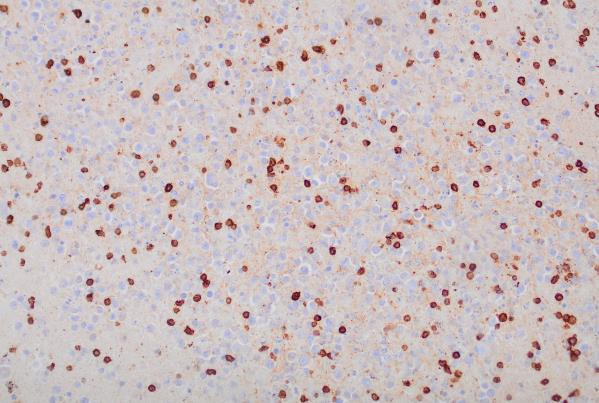
CD20 (200x magnification): Demonstrating B-cell marker expression.
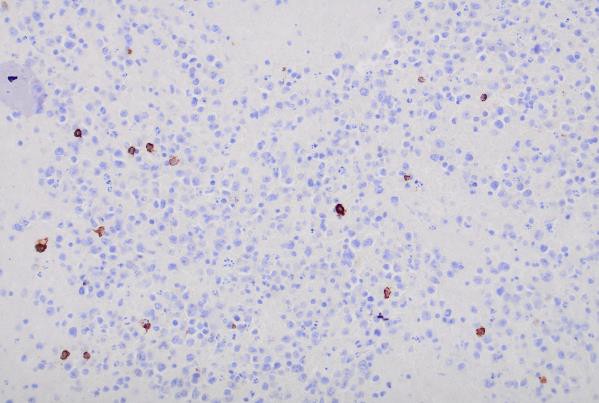
CD30 (400x magnification): Demonstrating variable CD30 expression in lymphoma cells.
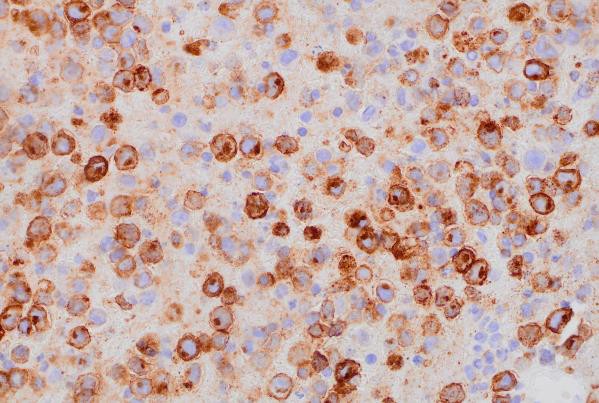
CD138 (400x magnification): Demonstrating plasma cell differentiation marker.
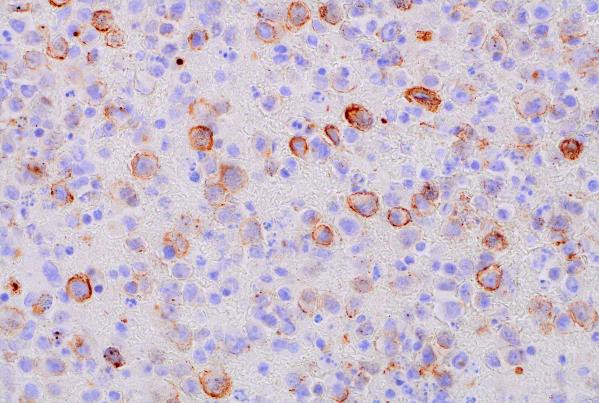
HHV-8 (400x magnification): Demonstrating HHV-8 positivity in tumor cells.
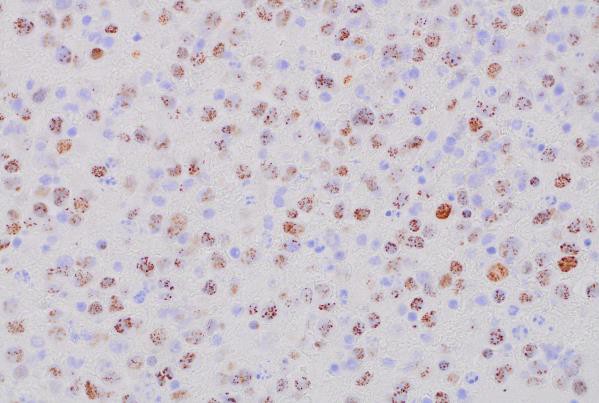
MUM1 (400x magnification): Demonstrating MUM1 expression in tumor cells.
Flow cytometry: Analysis of the cells within the lymphocyte gate shows 72% T lymphocytes and 3% B lymphocytes. The B cells are mature monoclonal B cells (CD19+CD20+CD22+) that express lambda light chains on their surface (kappa/lambda ratio: 1.2; normal range = 0.5-
4.0). The T cells are of mature phenotype and have a normal ratio of helper and cytotoxic T lymphocytes (CD4/CD8 = 1.0; normal ratio = 0.9-3.4). The majority of cells in the sample (60%) are mononuclear cells of the phenotype CD45+CD19-CD3-CD38+CD138+, which most closely corresponds to plasma cells.
A bone biopsy and puncture were also performed, revealing no evidence of infiltration from the underlying disease.
Antibody testing was conducted for Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, and hepatitis B and C. The test results were positive for EBV IgG and CMV IgG.
Discussion:
This case highlights the importance of considering Primary Effusion Lymphoma in the differential diagnosis of unexplained pleural effusions, particularly in elderly or immunocompromised patients. Primary Effusion Lymphoma is a rare and aggressive B-cell non-Hodgkin lymphoma associated with Human Herpesvirus 8 (HHV-8) infection. It is typically seen in immunocompromised patients, particularly in those with HIV/AIDS, but can also occur in elderly individuals with age-related immunosenescence. The differential diagnosis includes malignant mesothelioma, metastatic carcinoma, and other lymphomas (such as large B-cell lymphoma with plasmablastic features). The absence of CD20 expression and positivity for HHV-8 are key distinguishing features.
Key Learning Points:
- PEL presents as a malignant effusion without a detectable tumor mass.
- HHV-8 positivity is a defining feature and aids in diagnosis.
- It primarily affects immunocompromised individuals but can also occur in elderly patients.
- The prognosis remains poor despite chemotherapy, highlighting the need for novel therapeutic approaches.
Author of the case:
Ena Holjević, MD; Special thanks to assistant professor Christophe Štemberger,MD, PhD and assistant professor Irena Seili-Bekafigo, MD, PhD for their support in the developing of this case.
References:
Cesarman, E., Mesri, E. A., & Gershengorn, M. C. (2023). Primary Effusion Lymphoma. Diagnostics, 13(3), 370.
Coyle, T., Xie, Y., & Shergill, A. (2021). Primary Effusion Lymphoma: Current Perspectives. Frontiers in Oncology, 11, 33669719.