A 79-year-old male presented with a peri-rectal tumour measuring 20×16 mm without any additional clinical data. Endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNA) of the tumour was performed. The sample was stored in the cell medium and cytospins were prepared.
QUESTIONS
- Describe what you see.
- What does it represent?
- What is its significance?
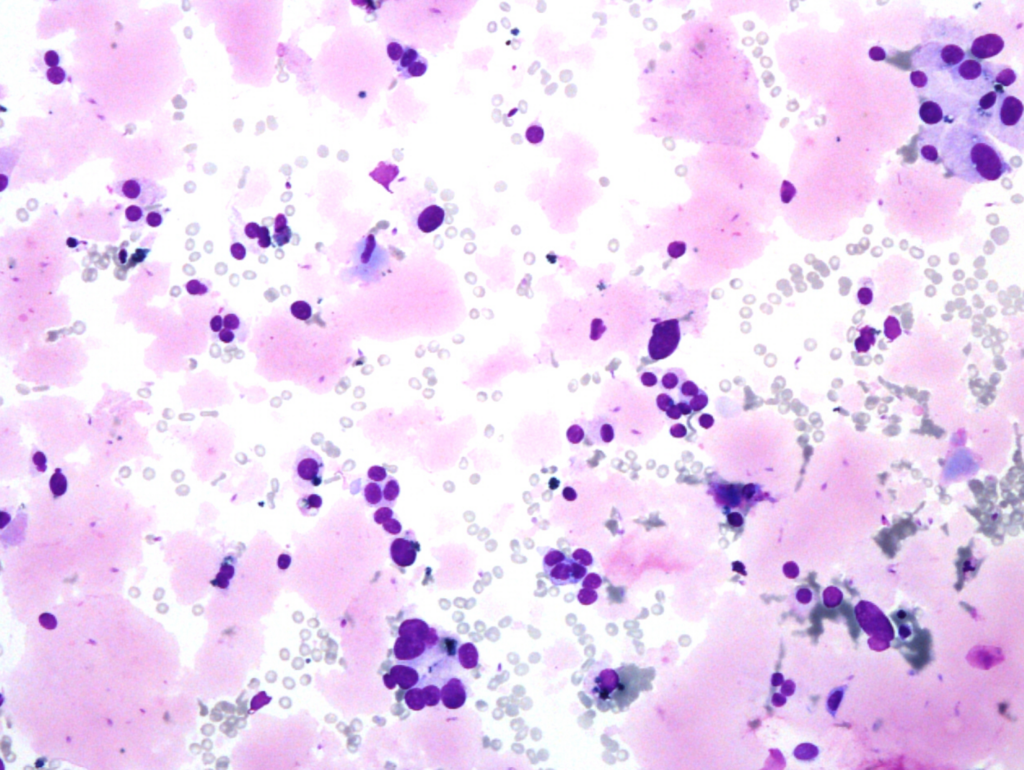
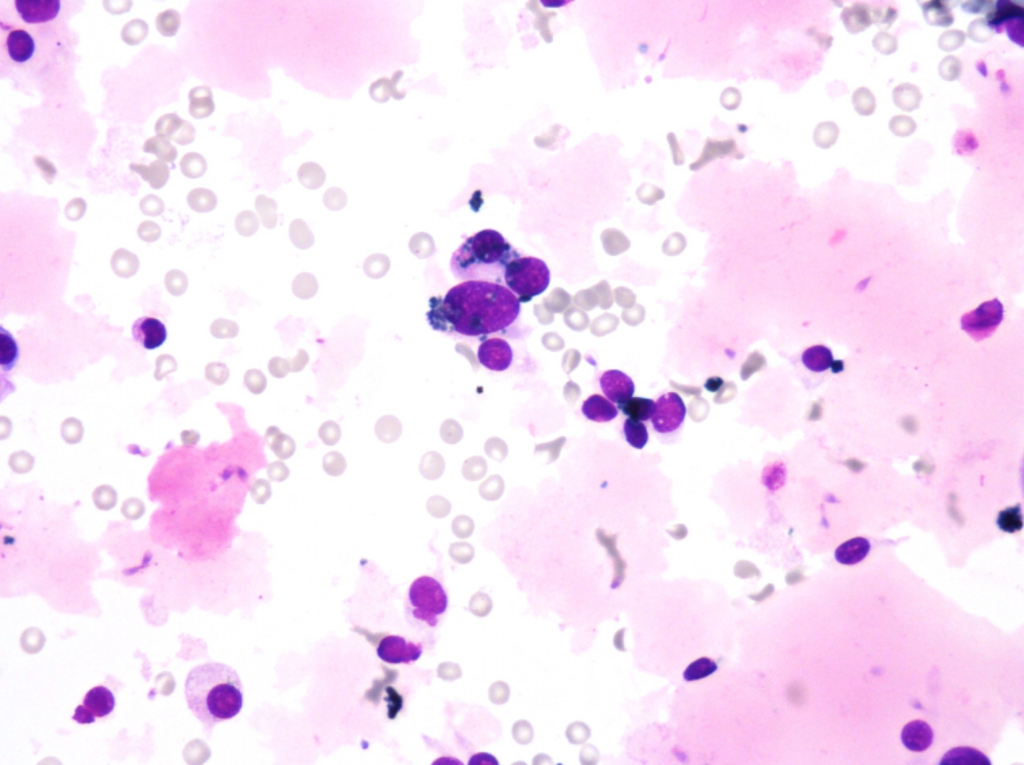
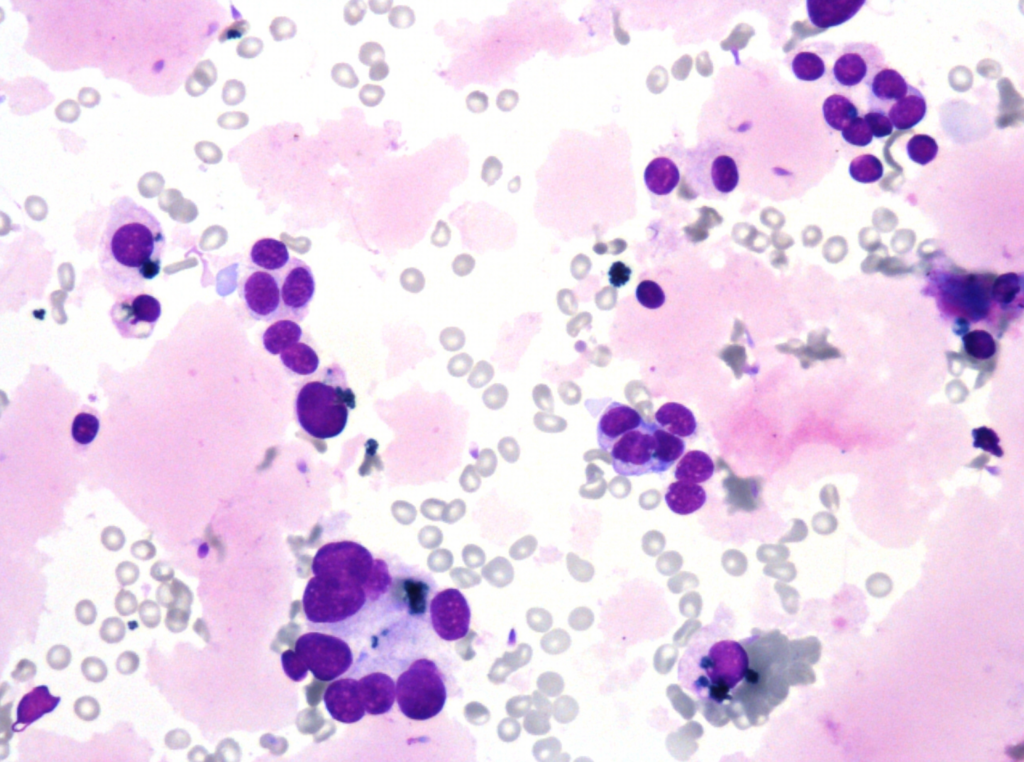
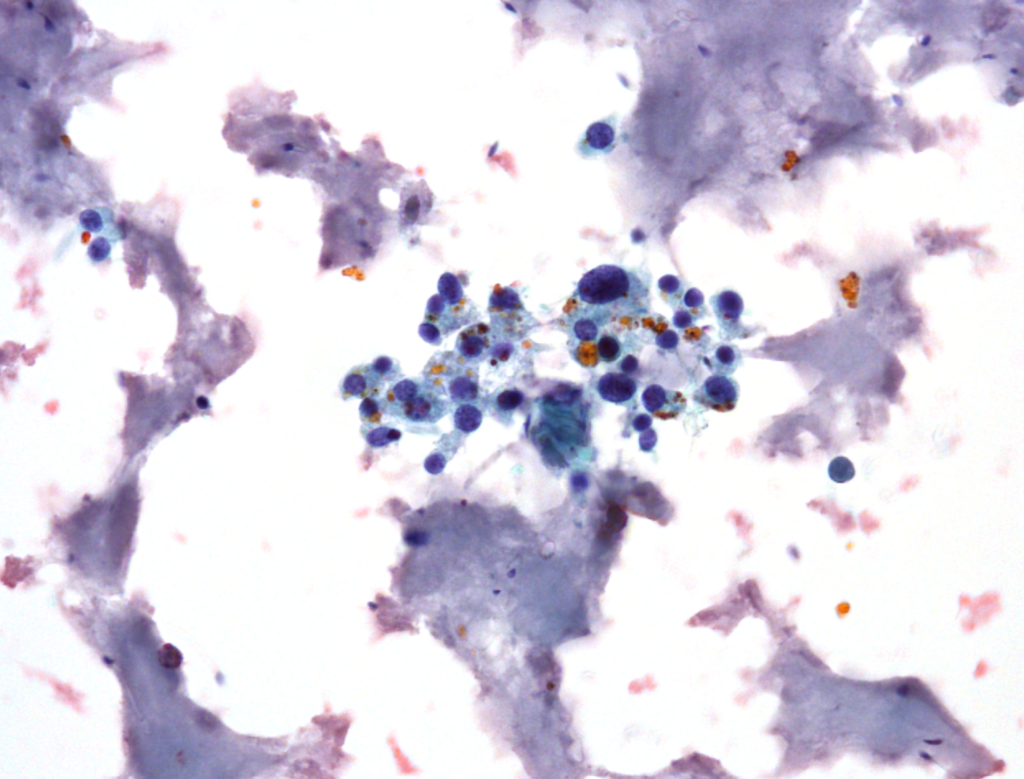
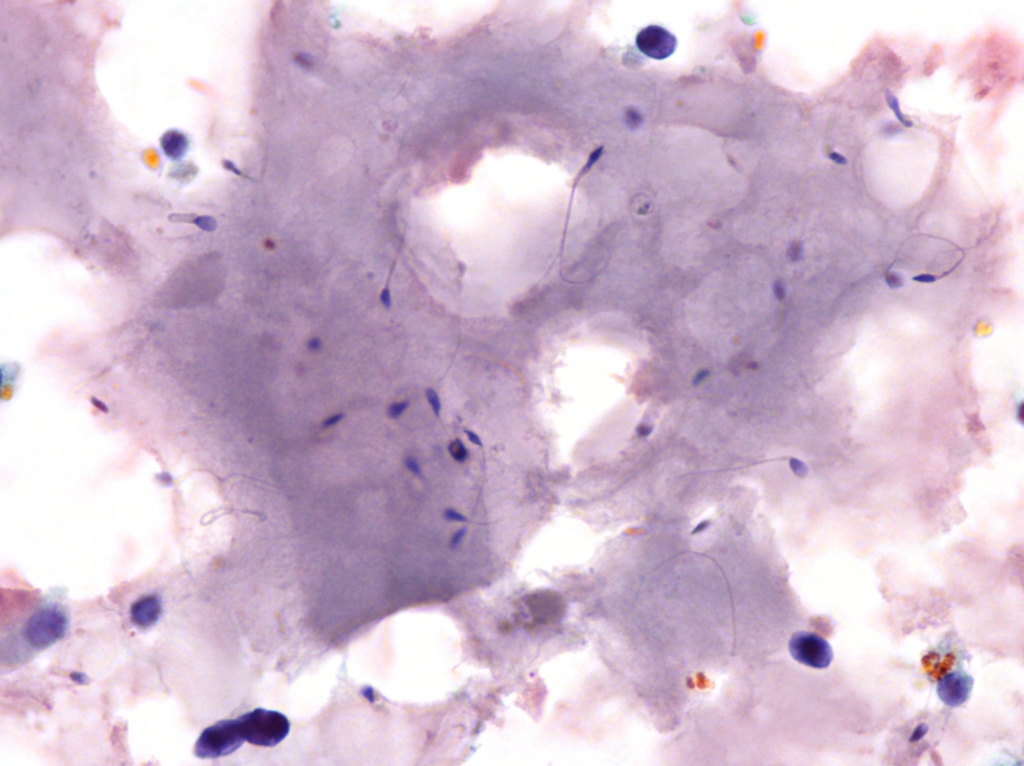
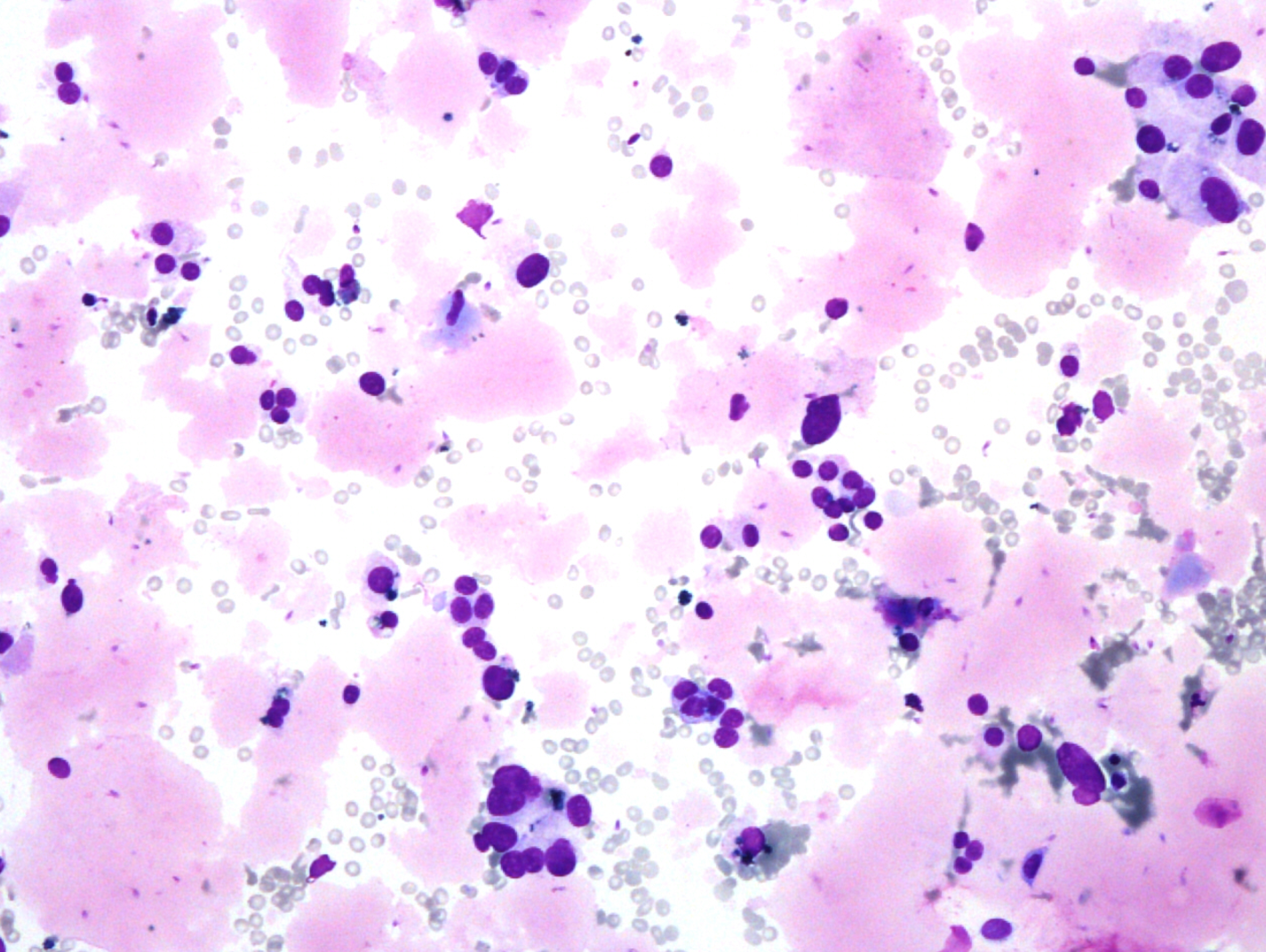
Fig. 5. Papanicolaou stain (PAP), x600
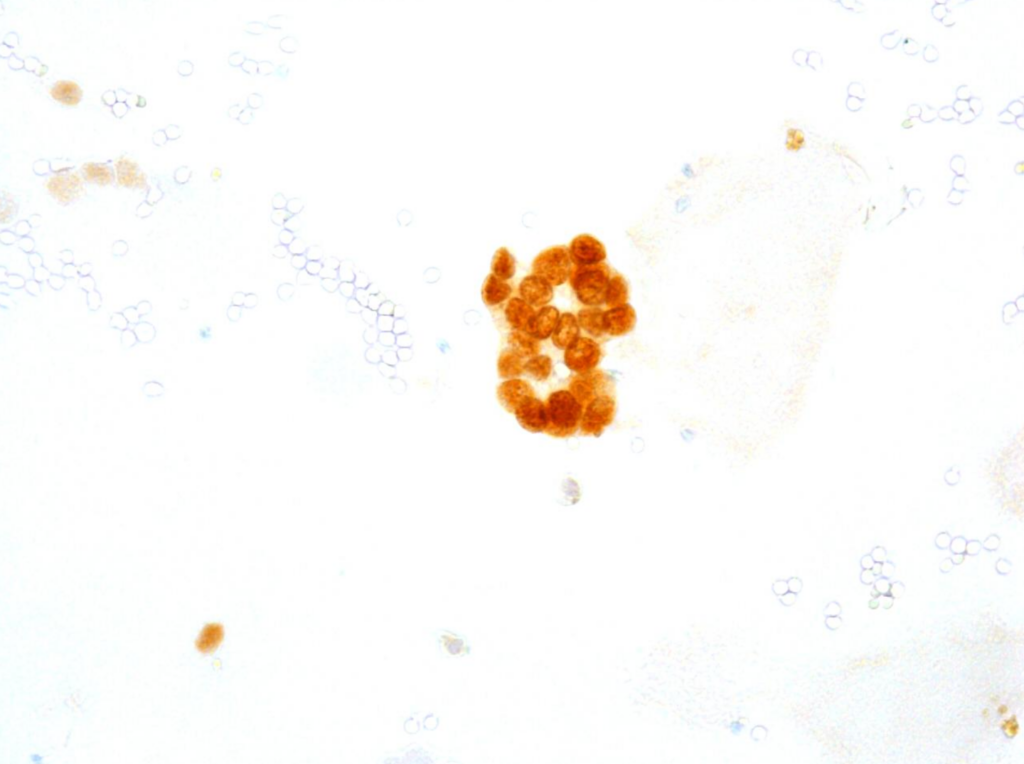
Fig. 6. Immunocytochemical reaction (ICC) PAX8, x400

Fig. 7. Immunocytochemical reaction (ICC) GATA3, x400
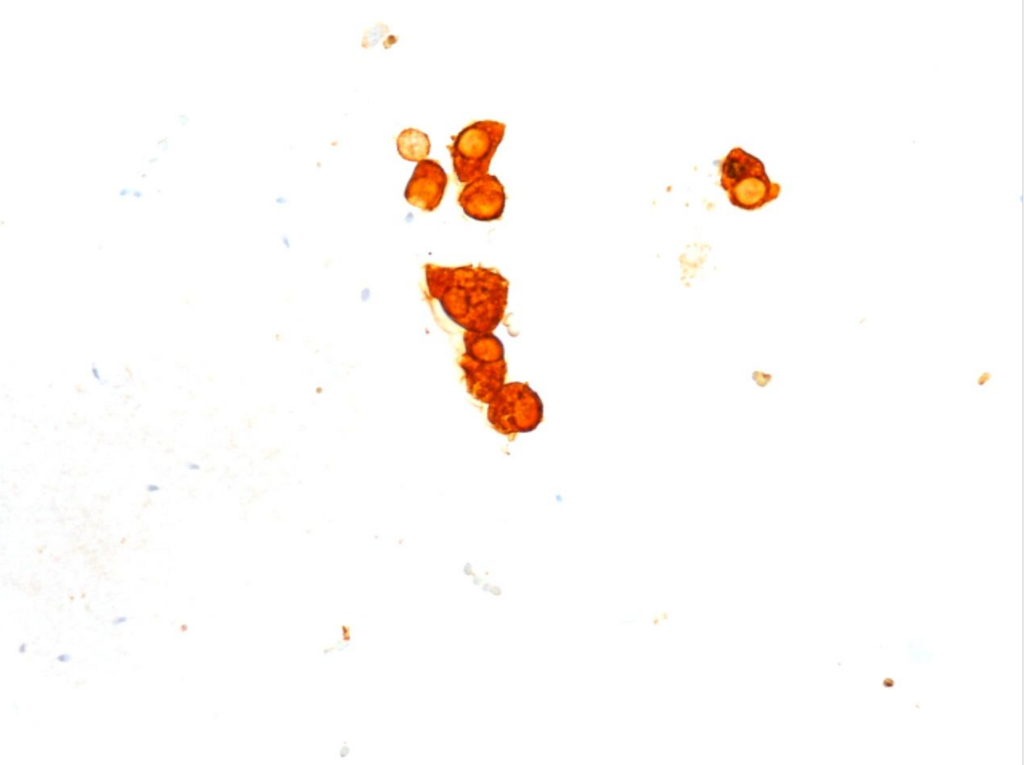
Fig. 8. Immunocytochemical reaction (ICC) CK5.6, x400
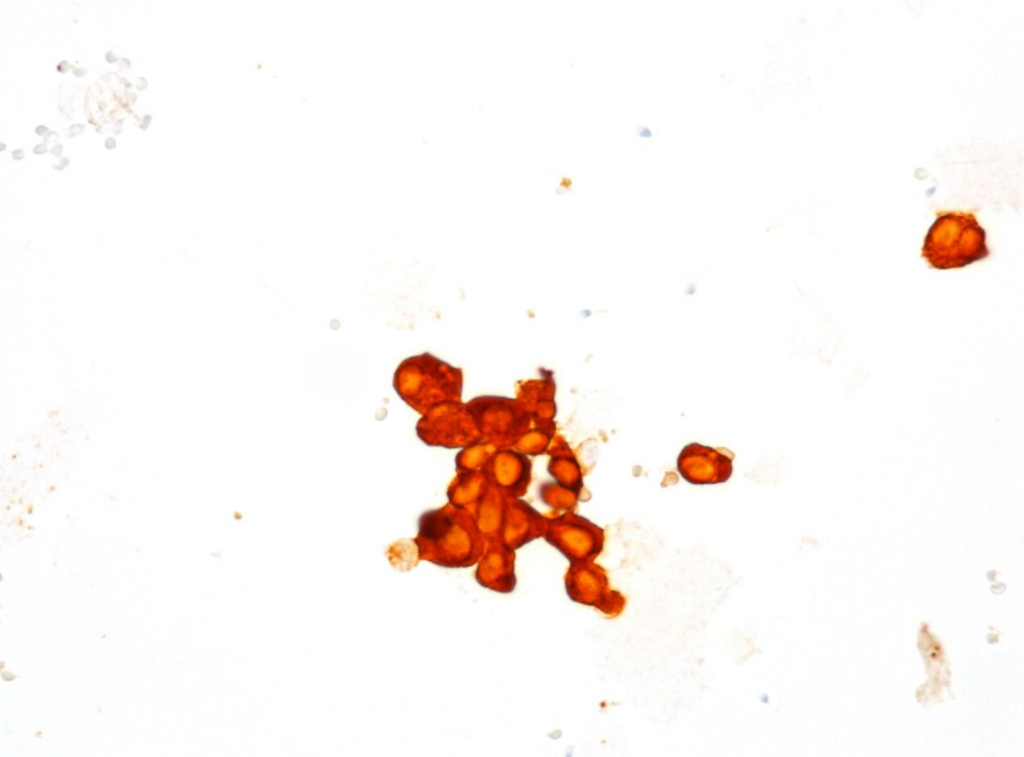
Fig. 9. Immunocytochemical reaction (ICC) CK7, x400
ANSWERS
a)
- predominantly dissociated cells forming discrete, small, poorly cohesive clusters
- moderately abundant, dense cytoplasm, focal coarse golden-brown pigment (Papanicolaou stain)
- oval and round nuclei, pronounced anisonucleosis, nuclear hyperchromasia, nucleoli
- abundant, thick proteinaceous material, sperms with long tails in the background
b) The cytomorphological picture and positive PAX8, GATA3, CK7, CK5.6 results and negative NKX3.1 and SATB2 immunocytochemical reactions were consistent with the diagnosis of a seminal vesicle.
c) Imaging studies prior to EUS-FNA showed that the most likely diagnosis was a peri-rectal soft tissue tumour. When a neoplastic process is suspected, and the epithelioid cell atypia is so pronounced, the differential diagnosis should include carcinoma, e.g., primary colon cancer, but also carcinoma arising from the surrounding organs, e.g., prostate, bladder, and kidney. The immunocytochemical reactions performed certainly excluded metastases of primary colon cancer and prostate cancer but not kidney or bladder cancer. While the cells were quite atypical, the presence of spermatozoa in the background of the sample, in combination with the coarse-grained golden-brown pigment (lipofuscin) in the cytoplasm were the key to the correct diagnosis.
Comment:
The EUS-FNA diagnosis of seminal vesicle raised the question of whether the sample was diagnostic, so comparison with the results of imaging studies was essential. In our case, after re-evaluation of the imaging studies, it was concluded that the peri-rectal lesion was a seminal vesicle because the imaging studies showed an association with the prostate.
Seminal vesicle sampling is infrequent in contemporary cytopathological practice. It may occur in patients with a history of previous abdominoperineal pelvic surgery or colorectal surgery because the seminal vesicles may be displaced posteriorly after abdominoperineal resection, giving the false appearance of a peri-rectal mass on radiologic imaging. However, our patient had no information about any previous surgeries, but this does not exclude them. The cytomorphology corresponding to the seminal vesicle may be very similar to residual colon adenocarcinoma after neoadjuvant chemoradiotherapy and resection. However, a negative SATB2 immunocytochemistry and a positive CK7 immunocytochemistry in combination with the cytomorphological picture can exclude the possibility of a primary colon adenocarcinoma.
Satturwar S, Monaco SE, Xing J, Brand RE, Pantanowitz L. Bizarre benign cells in peri-rectal endoscopic ultrasound-guided fine-needle aspiration due to seminal vesicle sampling. Diagn cytopathol. 2020;48(6):586-8.
Liaquat S, Idowu MO, Hatfield BS. Seminal vesicle adherent to rectal wall following neoadjuvant chemoradiotherapy: a potential false-positive diagnostic pitfall. Int J Surg Pathol. 2020;28(4):406-9.
Clinical case and pictures provided by:
Damjana Cimerman,